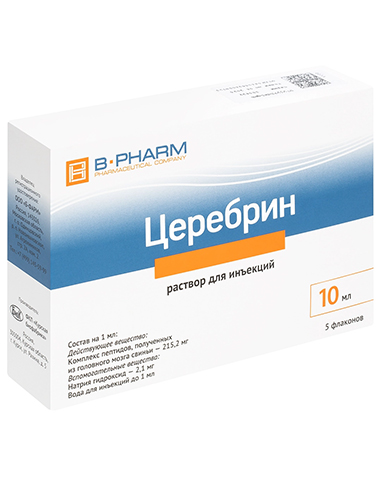
2mlx10pcs or 5mlx5pcs or 10mlx5pcs
The drug Cerebrin contains low-molecular biologically active neuropeptides that penetrate the blood-brain barrier and directly reach the nerve cells. The drug has an organ-specific multimodal effect on the brain, i.e. it provides metabolic regulation, neuroprotection, functional neuromodulation and neurotrophic activity.
Metabolic regulation: the drug Cerebrin increases the efficiency of aerobic energy metabolism of the brain, improves intracellular protein synthesis in the developing and aging brain.
Neuroprotection: the drug Cerebrin protects neurons from the damaging effects of lactic acidosis, prevents the formation of free radicals, increases survival and prevents the death of neurons under conditions of hypoxia and ischemia, reduces the damaging neurotoxic effect of excitatory amino acids (glutamate).
Neurotrophic activity: Cerebrin is a nootropic peptidergic drug with proven neurotrophic activity similar to the action of natural neuronal growth factors (NGF), but manifested under conditions of peripheral administration.
Functional neuromodulation: Cerebrin has a positive effect on cognitive impairment and memory processes.
Composition per 1 ml:
Active ingredient: a complex of peptides obtained from the pig brain 215.2 mg;
Excipients: sodium hydroxide 2.1 mg, water for injection up to 1 ml.
It is used parenterally. Doses and duration of treatment depend on the nature and severity of the disease, as well as the age of the patient. It is possible to prescribe single doses, the amount of which can reach 50 ml, but it is more preferable to conduct a course of treatment.
The recommended optimal course of treatment is daily injections for 10-20 days.
Acute conditions (ischemic stroke, traumatic brain injury, complications of neurosurgical operations): from 10 ml to 50 ml
In the residual period of cerebral stroke and traumatic injury to the brain and spinal cord: from 5 ml to 50 ml
In psychoorganic syndrome and depression from 5 to 30 ml
In Alzheimer's disease, vascular dementia and combined Alzheimer's-vascular genesis: from 5 to 30 ml
In neuropediatric practice: 0.1 - 0.2 mg / kg of body weight
To increase the effectiveness of treatment, repeated courses can be carried out until an improvement in the patient's condition is observed due to treatment. After the first course, the frequency of drug administration can be reduced to 2 or 3 times a week.
The drug Cerebrin is used in the form of injections: intramuscularly (up to 5 ml) and intravenously (up to 10 ml). Doses from 10 ml to 50 ml are recommended to be administered only by slow intravenous infusions after dilution with the proposed standard infusion solutions. The duration of the infusion is from 15 to 60 minutes.
If the injections are performed too quickly, a feeling of heat, increased sweating, and dizziness may occur. Therefore, the drug should be administered slowly.
The compatibility of the drug (within 24 hours at room temperature and with light) has been tested and confirmed with the following standard infusion solutions:
• 0.9% sodium chloride solution (9 mg NaCl/ml).
• Ringer's solution (Na+ - 153.98 mmol/l; Ca2+ - 2.74 mmol/l;
K+ - 4.02 mmol/l; Cl- - 163.48 mmol/l).
• 5% dextrose (glucose) solution.
It is allowed to prescribe Cerebrin simultaneously with vitamins and drugs that improve cardiac circulation, however, these drugs should not be mixed in the same syringe with Cerebrin.
It is necessary to use only a clear solution and only once.
Effect on the ability to drive vehicles and mechanisms
Cerebrin does not affect the ability to drive vehicles and mechanisms.
Contraindications
- hypersensitivity to the active or any of the excipients included in the drug;
- severe renal failure;
- epileptic status.
With caution
The drug is used with caution in case of allergic diathesis; diseases of an epileptic nature, including generalized epilepsy, due to a possible increase in the frequency of attacks; during pregnancy and breastfeeding.
Use during pregnancy and breastfeeding
Pregnancy
During pregnancy, Cerebrin should be used only after a thorough analysis of the ratio of the positive effect of treatment and the risk associated with its implementation. The results of experimental studies do not give grounds to believe that it has any teratogenic effect or toxic effect on the fetus. However, similar clinical studies have not been conducted.
Breastfeeding period
During the breastfeeding period, the drug Cerebrin should be used with caution, only after a thorough analysis of the ratio of the positive effect of treatment and the risk associated with its implementation.